Abstract
Cutaneous T-cell lymphoma (CTCL) is characterized by the expansion of malignant CD4+ T cells in the skin. There are two main subtypes of CTCL, mycosis fungoides (MF) and Sézary syndrome (SS). Previous studies have demonstrated defects in cell-mediated immunity, including altered cytokine profiles and decreased neutrophil function, and patients often have recurring bacterial and viral infections. Recent studies have shown increased expression of interleukin (IL)-15 in malignant CD4+ T cells in CTCL patients (Mishra et al., Cancer Discovery, 2016). Since IL-15 can enhance NK cell differentiation and activation, we hypothesized that NK cells from CTCL patients might have phenotypic and functional alterations due to these changes in homeostatic IL-15 levels.
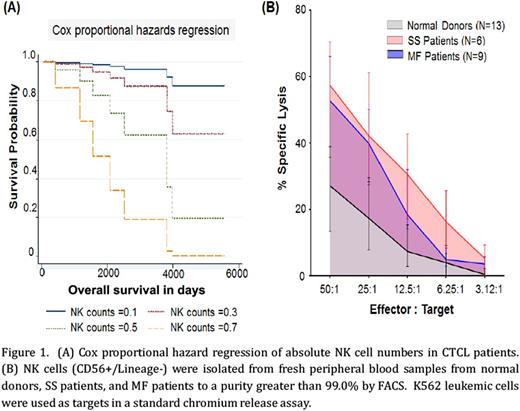
To evaluate the absolute number of NK cells in CTCL patients, CD56+Lineage- lymphocytes were quantitated by flow cytometry. SS patients had significantly fewer NK cells as compared to normal donors (mean ± SEM of absolute cell numbers in normal donors=0.2442 ± 0.02, n=51; SS=0.1072 ± 0.02, n=9; MF=0.2161 ± 0.01, n=112; unpaired t-test normal vs. SS, p=0.007). NK cell counts were significantly associated with overall survival, with the short-term risk of death increasing by 87.5% for every increase in 0.1 of absolute NK cell counts on univariate analysis utilizing the Cox proportional hazards model (Figure 1A; p=0.0413). We then examined the cytotoxic capacity of NK cells (CD56+Lineage-) freshly purified from both MF and SS patients by standard chromium release assay. There was a significantly higher level of specific lysis of tumor cells (K562 target cells) by NK cells from CTCL patients as compared to normal donors (Figure 1B, at 50:1 ratio mean ± SEM of percent NK cell cytotoxicity in normal vs CTCL = 27.47 ± 4.76, n=13 vs. 52.54 ± 4.88, n=12, unpaired t-test, p=0.001). Upon further transcript analysis of NK cell cytolytic mediators from CTCL patients compared to normal donors, we observed significantly increased levels of perforin (mean ± SEM of relative RNA in normal vs CTCL = 91.39 ± 9.18, n=3 vs. 485.7 ± 43.15, n=7, unpaired t-test, p=0.0004) and granzyme B (143 ± 23.79, n=3 vs. 431 ± 68.13, n=7 unpaired t-test, p=0.03). In addition, we observed increased levels of interferon-gamma and Fas Ligand (5.149 ± 0.4775, n=3 vs.13.93 ± 1.52, n=7; unpaired t-test p=0.007), indicating NK cells from CTCL patients exhibited consistently elevated activation patterns as compared to normal donor NK cells. We then examined the expression pattern of the IL-15 receptor complex on NK cells in CTCL patients as compared to normal donors by RNA sequencing. There was a significant elevation in IL-15Rα (mean ± SEM of relative RNA in normal vs CTCL = 0.8677 ± 0.1018, n=3 vs. 1.772 ± 0.2055, n=7; p=0.03) and IL-15Rγ (57.02 ± 7.525, n=3 vs. 154.7 ± 12.14, n=7; p=0.001), while IL-15Rβ was also elevated (128 ± 23.68, n=3 vs. 223.2 ± 25.43, n=7; p=0.056). Next we evaluated protein levels of phosphorylated signal transducer and activator of transcription 5 (p-STAT5) in fresh whole blood as the surrogate marker of IL-15 signaling. Preliminary findings demonstrate an increase in p-STAT5 in CTCL patients as compared to normal donors (mean ± SEM of fluorescent intensity in normal donors vs CTCL patients =1506 ± 90.82, n=5 vs. 1270 ± 50.59, n=4; unpaired t-test; p=0.07).
These data suggest that NK cell activation is enhanced in patients with CTCL, including MF patients with disease localized to the skin. Further investigations aimed at understanding the disconnect between NK cell numbers, activation, and disease control in CTCL patients are warranted.
Porcu: Kiowa: Research Funding; Miragen: Research Funding; Celgene: Research Funding; Tetralogic: Research Funding; Innate Pharma: Research Funding; Kura: Research Funding; Galderma: Research Funding; Cell Medica: Research Funding. William: Miragen: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal